Registration is Not the End! REACH24H Elaborates on Compliance Strategies for REACH Compliance
The European Chemical Agency (ECHA) is expected to release the Annual Evaluation Report 2015 in the forthcoming February. In 2015, ECHA has adopted new strategies for dossier compliance check, with special attention paid to substances that exert the largest impact on environmental and human health.
Given the increasingly frequent and strict evaluation activities taken by ECHA and its member states, REACH24H Consulting Group as the Only Representative (OR) of many non-EU companies has consistently represented the interests of our clients seeking for constructive communication with competent authorities and responded to multiple evaluation decisions and enforcement checks properly. It is worth noting that the post-registration compliance work can never be well accomplished without the cooperation of non-EU companies. In this article, REACH24H elaborates on ways for post registration compliance to address your concerns.
1. How to use the registration number by non-EU companies?
Please visit our NEWRSCC compliance platform to download the Submission Report issued by ECHA and the Confirmation Letter prepared by REACH24H when the registration number is available. Make sure that the contents of the Confirmation Letter are correct and remember to apply for a REACH Tonnage Coverage Certificate (TCC) at the NEWRSWCC platform each time when a deal is made. Confirm if you are obliged to pass the SDS or eSDS to downstream users. Besides, it is always necessary to maintain a good communication between OR and importers and follow closely any updates concerning this substance under EU REACH.
2. What are the possible official moves after the completion of registration?
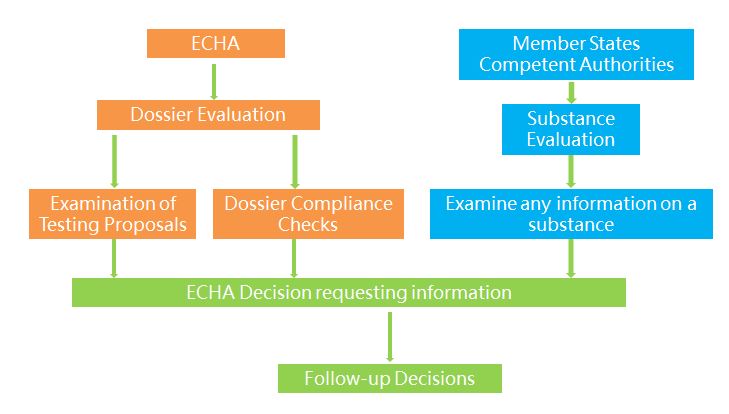
The two possible moves taken by ECHA or member states after the completion of registration are evaluation and compliance check. There are two types of evaluation, including dossier evaluation and substance evaluation. ECHA evaluates the information submitted by companies to examine the quality of the registration dossiers and the testing proposals and to clarify if a given substance constitutes a risk to human health or the environment. If the substance is considered as possibly posing potential risks and dangers, then a Member State will designated to conduct substance evaluation and examine if it constitutes a risk to human health or the environment.
Compliance checks mainly involve specific importers. Competent authorities of the importing countries evaluate the substance identity description and the safety information in the dossier including the chemical safety report or specific parts of the dossier.
Please note that if you receive any decisions after substance evaluation or compliance check, a timely reply before the official deadline is a must, otherwise the registration number can be invalid.
Evaluation Process
3. Are your substances subject to evaluation?
1) Dossier evaluation. ECHA may examine any registration dossier to verify if the information submitted by registrants is compliant with the legal requirements. Dossier selection for compliance check is either random or concern based (targeted). According to the new strategies taken by ECHA in 2015, priority has been given to substances of high risks, including two categories: one is substances registered at high tonnage band with many data gaps and the other is substances with high exposure to workers, customers and the environment.
2) Substance evaluation. Member States evaluate certain substances to clarify whether their use poses a risk to human health or the environment. The selected substances are listed by ECHA in the community rolling action plan (CoRAP) following the opinion of the Member State Committee. An evaluating Member State will be designated for each substance on the final CoRAP. Listed in the CoRAP are mostly Environment/Suspected PBT and Human Health/CMR substances.
4. What are the focuses of inspections carried out by Member States?
Based on the practical experiences of dealing with inspections by the Health and Safety Authority (HSA), the lead Competent and Enforcement Authority for REACH in Ireland, REACH24H concluded the following major concerns:
- Substance and tonnage band information corresponding to the registration number.
- Signed Power of Attorney.
- Tonnage coverage records by importers.
- Safety Data Sheet management.
- Intermediate and exemption validity.
Please refer to the previous article written by REACH24H for more information:
According to the news released by ECHA on 26 June, a fifth coordinated enforcement project (REF-5) will be prepared and executed focusing on obligations related to extended safety data sheets (e-SDSs), exposure scenarios, risk management measures and operational conditions, indicating that inspections may be carried out in a more strict manner.
5. What to do after receiving the registration documentation from OR?
REACH24H follows closely any evaluation or inspection decisions passed by ECHA, the Member States and LR and delivers such important information to you in the form of a SIEF report. The decisions issued are more often than not lengthy and complicated, involving hazardous information of the substance, regulatory requirements and the provisional basis of the decision etc. The decisions can be English documents including tens of or even hundreds of pages. To help you understand the decision well, REACH24H experts will formulate a SIEF report on the decision based on our regulatory compliance experiences and professional expertise to facilitate a cost-effective way for compliance.
Here is a typical case successfully handled by REACH24H. After the registration of furfuryl alcohol in 2013, REACH24H has been in close contact with the LR. As this substance potentially constitutes risks of carcinogenicity and toxicity, the Poland competent authority issued a decision for substance evaluation of this substance. The LR organized over 10 teleconferences or on-site meetings to discuss coping strategies and proposed the establishment of an international Furfuryl Alcohol consortium to address the regulatory issues. After a detailed analysis of the coping strategies of LR with the actual uses and risk control measures of downstream users well taken into consideration, REACH24H concluded that it was not necessary to join the consortium and helped our clients avoid follow-up sharing of testing costs.
Please read carefully the SIEF report sent to you by REACH24H, which will help you a lot in working out compliance strategies. If the product receiving decisions is pivotal to your trade in the EU market, you can be rest assured that we are always readily to help you communicate with the authorities or LR representing your interests.
Strict regulation has been taken over chemicals imported to the EU market in line with EU REACH to protect human health and environmental safety. Registration is just a first step to open the EU market. Companies need to cooperate actively when official evaluation and inspections are carried out. REACH24H is well positioned to help you get access to and secure a good place in the EU market at the prerequisite of compliance with EU REACH.
If you’re interested in more details about this topic, please send your queries directly to customer@reach24h.com .
