ECHA Plans to Conduct Substance Evaluation on 28 Substances, Including Carbon Black
On March 19th, 2024, the European Chemicals Agency (ECHA) announced its Community Rolling Action Plan (CoRAP) for the period 2024-2026. Based on the opinions of the Member State Committee (MSC), ECHA has selected 28 substances, including carbon black and benzaldehyde, as substances of potential concern and plans to assess them in batches over a period of three years. The purpose of these evaluations is to enhance the regulatory oversight and safety of the respective substances.
What is CoRAP?
The European Union’s REACH regulation requires ECHA to regularly identify substances that are suspected of posing risks to human health or the environment and to clarify these risks through subsequent substance evaluations. Therefore, based on information on the hazardous properties of substances (e.g., CMR, PBT, endocrine disruptors) and exposure-related information (including exposure risks and total tonnage), ECHA and its Member States annually determine and update a list of substances to be prioritized for evaluation over the next three years. The list is known as the Community Rolling Action Plan, abbreviated as CoRAP.
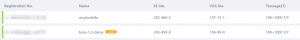
All substances listed in CoRAP have been synchronized and marked in REACH24H’s Supply Chain Compliance platform, RSCC. Companies can log in to the RSCC system and check whether the substances they registered are listed in the CoRAP inventory under the “Registered Substances” section.
If a substance is included in the CoRAP list on ECHA’s website, it means that EU Member States have already been evaluating or will evaluate the substance in the coming years.
What happens when a substance is included in CoRAP?
Once a substance is included in CoRAP, the designated Member State has one year to evaluate the substance and, if necessary, issues a draft decision requesting further information from the registrants of the substance to clarify potential risks of concern. This implies that additional costs are likely to be required for new data/information to ensure the continued validity of the registration for that substance.
Typically, ECHA notifies all registrants, including the Lead Registrant (LR), of the substance evaluation decision. Registrants need to submit the information required in the decision within the specified timeframe, which often includes higher-tier experimental data and sometimes, requirements that exceed REACH Annex VII to X standard. It is important to note that all registrants are required to share the costs of data supplementation, irrespective of their registered tonnage or registration type.
What can lead to an increase in registration fees?
1) Substance evaluation by ECHA
2) Dossier evaluation by ECHA, including Compliance Check (CCH) and Testing Proposal evaluation (TPE)
Unlike the substance evaluation that focuses on the risks associated with a substance, the purpose of dossier evaluation is to review the completeness and compliance of the registration dossier submitted by the Lead Registrant (LR) on behalf of the JS. This includes assessing whether the submitted registration data meets the latest REACH requirements and whether the testing proposals submitted by LR are appropriate and practical. The cost of data supplementation resulting from this evaluation is related to the registered tonnage and registration type. In other words, the registrants under the corresponding tonnage need to share the cost of supplementing the respective data requirements. (See Dossier Evaluation Status on ECHA’s website)
3) LR-spontaneous updatesAccording to Article 22(1) of the REACH regulation, registrants are required to proactively update their registration dossiers when they become aware of any new changes or information. Therefore, some LRs may voluntarily review the data quality and gaps in the original dossier periodically, before the official evaluation is initiated. This results in voluntary data gap filling and registration updates and the associated costs shall be shared among the relevant joint registrants.
4) Recalculation of Letter of Access (LOA) fees and administrative costs
Some LRs may recalculate the registration costs within a certain period. In many cases, the previous registrants may need to contribute additional fees to cover the costs associated with official evaluations, SIEF (Substance Information Exchange Forum) operation, and accumulated management costs over the years.
How to effectively avoid and reduce the additional costs?
Companies need to assess their actual trade export volume, which refers to the total amount of exported goods that have arrived within the EU, including direct and indirect exports.
- No exports in recent years, with an export volume of 0
It is recommended to promptly notify ECHA of the cessation of manufacture to avoid potential risks of cost supplementation in the future. It should be noted that any costs incurred before the cessation of manufacture still need to be paid. In principle, according to the REACH regulation, companies are required to notify the authorities within three months after ceasing manufacture /import.
- Actual export volume significantly lower than the registered tonnage
It is recommended to promptly notify ECHA of the reduction in registered tonnage, which can help mitigate the risks of data supplementation costs associated with dossier evaluation, LR-initiated updates, LOA fee recalculations, and other factors.
- Actual export volume and registered tonnage are in the same tonnage range
When receiving draft decisions from ECHA regarding substance evaluation or dossier evaluation, it is important to conduct a cost-benefit analysis based on estimated expenses, future trade situations, risk tolerance, etc. This will enable timely decision-making and preparation for cost supplementation or the avoidance of obligations in response to the decisions.
FAQs
Q1: What should I do if the export volume increases after reducing the registered tonnage?
A: After reducing the tonnage, if the export volume increases, you can upgrade the tonnage based on the actual import and export situation. Depending on the specific circumstances, you may need to pay administrative fees to ECHA, data fees, and dossier update fees:
- Administrative fees to ECHA
If you upgrade the tonnage back to the original registered tonnage, no additional administrative fees will be charged. However, if the upgraded tonnage exceeds the original registered tonnage, you will need to pay the difference in administrative fees.
- Data fees and dossier update fees
Before upgrading the tonnage, it is necessary to confirm with the Lead Registrant (LR) whether there will be any additional costs associated with the upgraded tonnage, including but not limited to data supplementation fees and management fees. If there are additional costs, the company still needs to complete the payment and perform dossier updates.
Q2: Since it is possible to abandon the registration number after receiving the draft decision, can I make the decision until the draft decision is issued, despite there are currently no exports?
A: The draft decision stage applies only to substance evaluation and dossier evaluation issued by ECHA. For LR-spontaneous update, LOA fee recalculations, and administrative fee collections, LR assumes that joint registration members agree to share the corresponding costs, and in most cases, LR does not provide advance notice for decision-making. Therefore, if there are currently no exports, it is recommended to promptly notify the authorities of the cessation of manufacture to comply with the requirements of the REACH regulation and reduce the risk of cost supplementation.
REACH24H advises companies to continue fulfilling their obligations for updates and data supplementation even after completing the REACH registration to maintain ongoing compliance with their registration numbers. REACH24H will also continue to monitor official evaluation plans and updates and notify relevant companies in a timely manner to facilitate informed decision-making and timely responses, thereby avoiding unnecessary compliance risks. If you need any help, please feel free to contact us via rcuhelp@reach24h.com or customer@reach24h.com.