Product Registration
1. DMF Filing for APIs, Pharmaceutical Excipients and Packaging Materials
1.1 Legal basis
- Drug Administration Law of the People’s Republic of China (2019 Revision);
- Provisions for Drug Registration;
- Announcement No. 146 of CFDA, 2017: Announcement of Adjustment on the Review and Approval Procedure of APIs, Pharmaceutical Excipients and Packaging Materials;
- Announcement No. 56 of NMPA, 2019, Announcement of Improvement on the Separated Filing and Linked Review System of Drug Products and the Supervision.
1.2. Product scope
APIs, pharmaceutical excipients and packaging materials which are developed, produced, imported and used within the territory of the People’s Republic of China.
1.3 Applicant qualification
Domestic manufacturers of APIs, pharmaceutical excipients and packaging materials. Overseas manufacturers or holder of APIs, pharmaceutical excipients and packaging materials. For overseas applicants, a Chinese business entity must be appointed as local agency.
1.4 Filing type and procedure
- APIs filing, change management and annual report;
- Pharmaceutical excipients filing, change management and annual report;
- Packaging materials filing, change management and annual report.
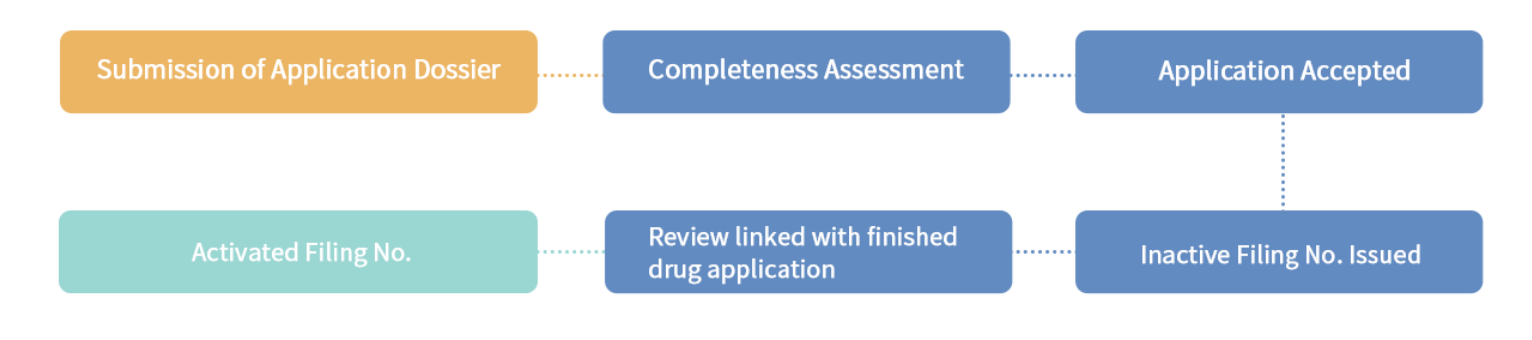
1.5 Post-marketing obligation
- Change management, including major, medium, minor-level changes and basic information change;
- Annual report.
1.6 Our service
1) Authorization to act as local agent for product filing;
2) Support for application materials checklist and data gap analysis for feasibility assessment;
3) Customized risk evaluation and optimized registration strategy analysis to avoid possible deficiencies in application dossier and to minimize risk of rejection;
4) Document translation and dossier preparation;
5) For high-risk products, assistance in sample testing and NMPA on-site inspection –on-site pre-audit or mock inspection can be arranged;
6) All-the-way consulting services for DMF filing, including communications with CDE reviewers, reply to deficiency letters from CDE, prepare and submit supplementary application;
7) Extended service coverage after market approval, including preparation and submission of annual reports, applications for change management.
2. Application for Market Approval of Generic Drugs: ANDA
2.1 Legal basis
- Drug Administration Law of the People’s Republic of China (2019 Revision);
- Regulations for the Implementation of the Drug Administration Law of the People’s Republic of China (2019 Amendment);
- Measures for the Administration of Drug Registration (2020 Revision)
2.2 Product scope
Generic drugs which are imported, sold and used within the territory of the People’s Republic of China.
2.3 Eligibility of Applicants
Domestic applicants shall be an enterprise or drug development institution that can assume corresponding legal responsibilities. Overseas applicants shall be overseas pharmaceutical manufacturers with legal drug market approval. For overseas applicants, they must entrust a China business entity as the local agency.
2.4 Application procedure
Domestic generic drugs, imported generic drugs
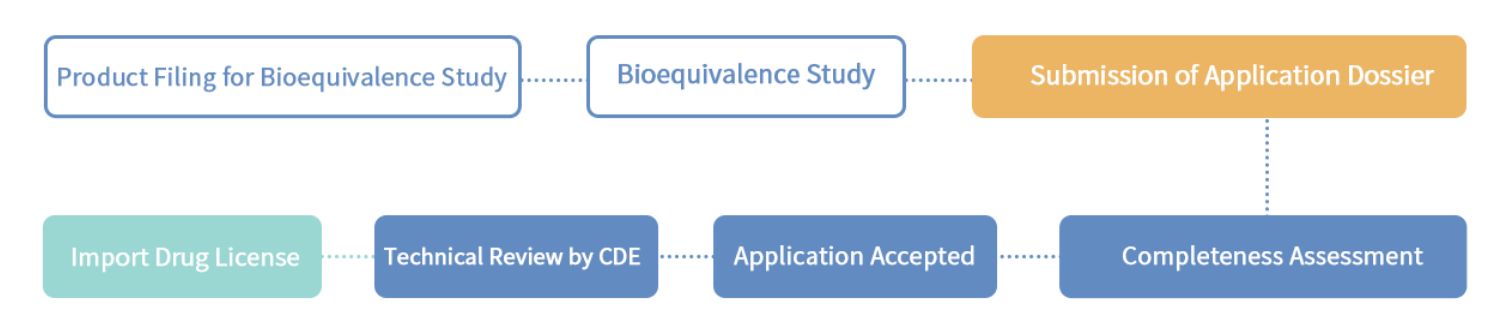
2.5 Post-marketing obligation
- Change management, including major, medium, minor-level changes and basic information change;
- Annual report.
2.6 Our Service
1) Authorization to act as local agent for product filing;
2) Support for application materials checklist and data gap analysis for feasibility assessment;
3) Customized risk evaluation and optimized registration strategy analysis to avoid possible deficiencies in application dossier and to minimize the risk of rejection;
4) Support in communications with CDE reviewers;
5) Support in sample testing and NMPA on-site inspection –on-site pre-audit or mock inspection can be arranged;
6) Navigate clinical trials or bioequivalence (BE) study in all stages of development, design and implementation: provide experimental design and implementation planning, assistance for Clinical Research Organization (CRO) search in China, assessment and due diligence of CROs;
7) Translation of documents for non-clinical studies, including pharmacology & toxicology documents, reference journal articles etc.
8) All-the-way consulting services for drug registration until approval, including communications with CDE reviewers, reply to deficiency letters from CDE, preparation and submission of supplementary applications;
9) Extended service coverage after market approval, including preparation and submission of annual reports.
3. Application for Clinical Approval: CTA/IND, BLA, BE studies and MRCTs
3.1 Legal Basis
- Drug Administration Law of the People’s Republic of China (2019 Revision);
- Regulations for the Implementation of the Drug Administration Law of the People’s Republic of China (2019 Amendment);
- Measures for the Administration of Drug Registration (2020 Revision);
- Announcement No. 50 of NMPA, 2018, Announcement on the Adjustment of Review and Approval Procedures for Clinical Trials of Drugs.
3.2 Registration Scope
Clinical trial application for drug market approval within the territory of the People’s Republic of China.
3.3 Eligibility of Applicants
An applicant shall be an enterprise or drug development institution that can assume corresponding legal responsibilities. Overseas applicants must entrust a China business entity as their local agency.
3.4 Application Procedure
Clinical trial application of innovative new drugs and improved new drugs
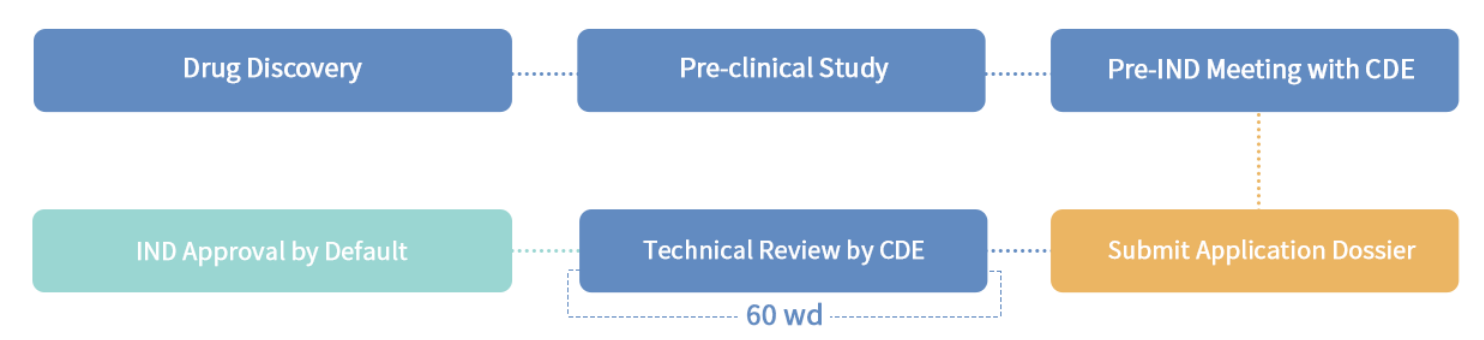
3.5 Our service
- Application for Pre-IND meeting and preparation of relevant meeting materials;
- Feasibility assessment/data gap analysis for the IND application to avoid possible deficiencies in application dossier and to minimize the risk of rejection;
- Preparation of the outline of IND application dossier;
- IND application dossier reviewing work, translation, writing and submission in CTD format; e-CTD document editing and format conversion;
- Testing sample, testing application, progress tracking and support;
- All-the-way consulting service for IND application until approval, including communications with CDE reviewers, reply to deficiency letters from CDE, preparation and submission of supplementary applications.
4. Application for Market Approval of New Drugs: NDA
4.1 Legal basis
- Drug Administration Law of the People’s Republic of China (2019 Revision);
- Regulations for the Implementation of the Drug Administration Law of the People’s Republic of China (2019 Amendment);
- Measures for the Administration of Drug Registration (2020 Revision).
4.2 Product scope
Market approval of New drugs within the territory of the People’s Republic of China.
4.3 Eligibility of Applicants
An applicant shall be an enterprise or drug development institution that can assume corresponding legal responsibilities. Overseas applicants will must entrust a China business entity as their local agency.
4.4 Application Procedure
Application for market approval of innovative new drugs and improved new drugs
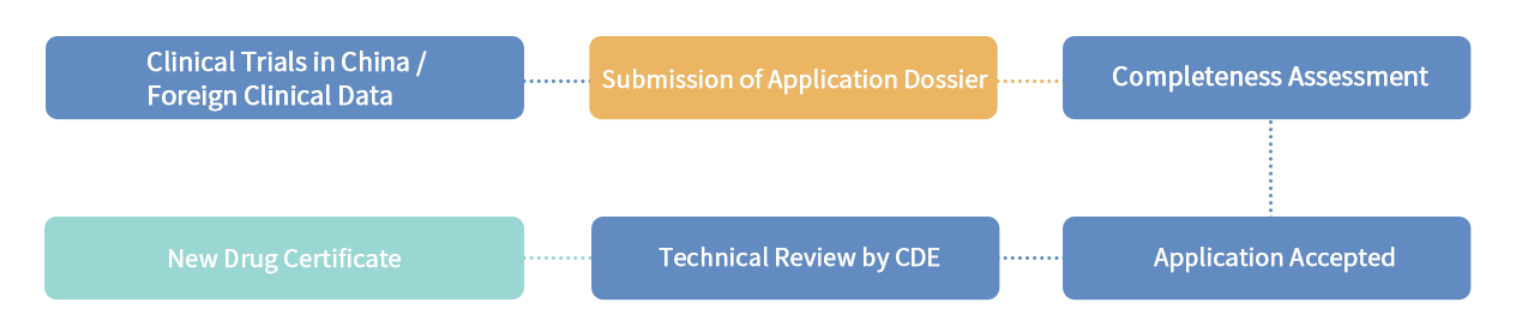
4.5 Post-marketing Obligation
- Change management, including major, medium, minor-level changes and basic information change;
- Annual report.
4.6 Our Service
- Feasibility assessment or data gap analysis for the NDA applications to avoid possible deficiencies in application dossier and to minimize the risk of rejection;
- Preparation of the outline of NDA application dossier;
- NDA application dossier review, translation, drafting and submission in CTD format;
- All-the-way consulting service for NDA application until approval, including communications with CDE reviewers, reply to deficiency letters from CDE, preparation and submission of supplementary applications;
- Change in application services after market approval;
- Editing and submission of annual reports.


