EU to Make More Information on Biocidal Products Available
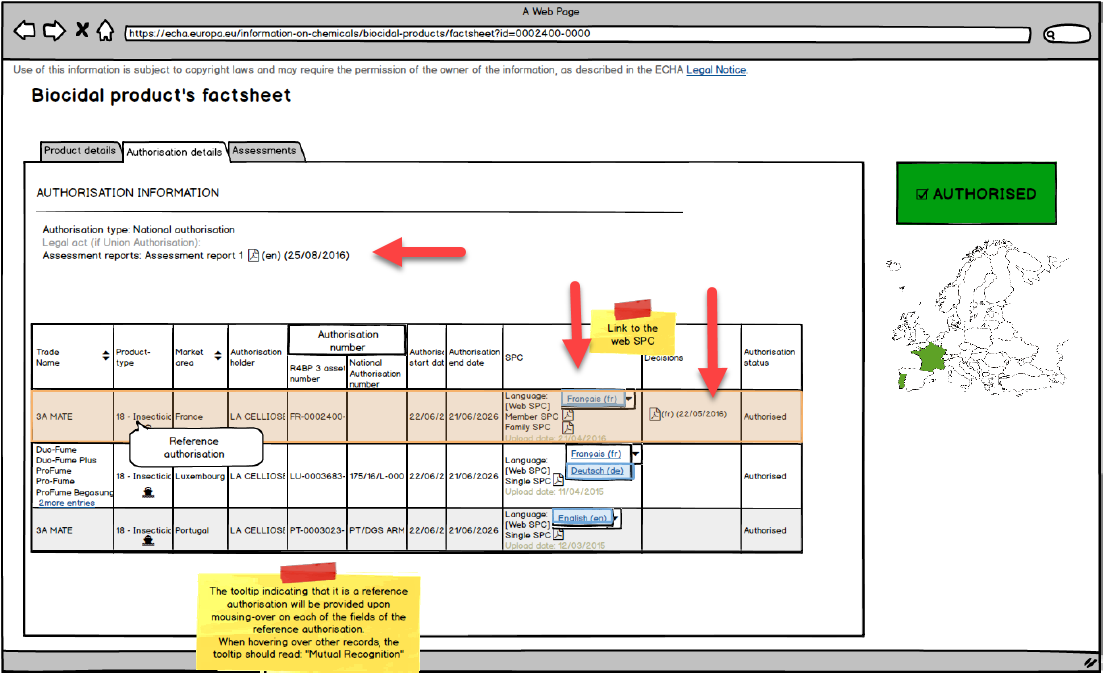
Website preliminary model of
This move by ECHA is aimed to improve transparency in the process of compliance, to some extent serving as a tool for market oversight and compliance promotion, although there is a potential risk of data being leaked. To this end, ECHA, EU Member States and applicants will have to reach consensus as to what is considered “non-confidential data”. Initially, when submitting an application, the applicant will have to determine the level of confidentiality for all their data. Later on, when the assessment is coming to an end, the applicant will have to make said information public. The authorized evaluation authority will be responsible for checking the confidential and non-confidential information of the product assessment report in the final stage of the evaluation.
The competent evaluating authority is also responsible for reviewing and ensuring all information on an authorized product to be made public is available by September 30, 2018. The company should actively communicate with this authority, complete all requirements –such as modified document name–and make it clear that the information to be published does not include any confidential information pertaining to the company.
